Merck's new anabolic steroid: MK-0773 (J Biol Chem. 2010 May 28; 285 (22): 17054-64.)
He doesn't have a real name yet, but if it is up to the important men of Merck / Schering-Plow Pharma , then it will be on the market in the near future: the anabolic steroid MK-0773. You can see his structural formula below.
In fact, experts expected that the S4 already available through websites would conquer the medical market for muscle, bone and libido enhancers. Or else a chemical brother of S4 that comes from the same tube: the GTx company. However, developments in the market for new steroids suggest that the era of steroids has not yet ended.
GTx was founded by the chemists who were the first to make new steroids without a steroid structure. SARMs are called those things: they interact with the androgen receptor, but have hardly any androgenic effects.
GTx collaborated with Merck, but when Merck merged with Schering-Plow, that collaboration ended. If you rely on press releases, GTx will now continue on its own with its own SARMs. [tradingmarkets.com June 11, 2009] [Offline] Merck, who is led by new bosses, has taken up research into the old-fashioned steroids again.
Well, anabolic steroids have a bad name. That is why the Merck researchers pretend that their MK-0773 is also a SARM. SARM is simply a more positive term than 'anabolic steroid'. You can't blame the Mercenaries. Even trenbolone has SARM-like properties, some researchers claim in recent reviews. [Steroids. 2010 Jun; 75 (6): 377-89.]
In the Journal of Biological Chemistry, researchers from Merck Research Laboratories publish results of trials with MK-0773 on rats. They still had the 4-aza-steroid somewhere. The researchers discovered that the thing had an interesting effect when they subjected a whole batch of compounds to a new test. That test determines whether compounds could do fun things with the androgen receptor without causing too many side effects.
DHT, the researchers discovered, binds to the androgen receptor 6 times better than MK-0773. The killeranabolic methyl trenbolone even binds to the androgen receptor 8 times better than MK-0773.
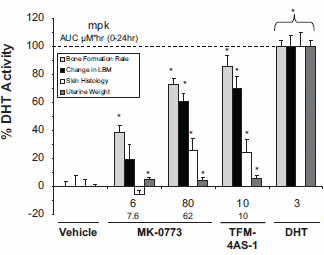
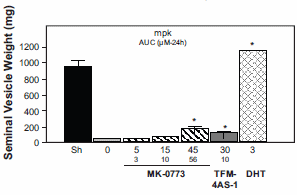
Nevertheless, MK-0773 increases the lean body mass in rats without testes. Although you need more mg / kg for this than if you use DHT, the androgenic side effects - measured by the effect on the seminal vesicles - do not represent anything.
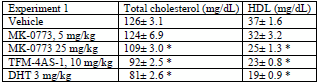
Lowering cholesterol did MK-0773 to a lesser extent than DHT.
At this moment you will find 1 publication about the new drug on PubMed, but Merck has now completed 3 human trials with oral versions of the drug. [clinicaltrials.gov] This also includes trials that compare MK-0773 with good old testosterone. We postpone our assessment of MK-0773 until those studies are published.
Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity.
Yarrow JF1, McCoy SC, Borst SE.
Author information
1Geriatric Research, Education & Clinical Center, VA Medical Center, Gainesville, FL 32608, United States. [email protected]
Abstract
Recently, the development of selective androgen receptor modulators (SARMs) has been suggested as a means of combating the deleterious catabolic effects of hypogonadism, especially in skeletal muscle and bone, without inducing the undesirable androgenic effects (e.g., prostate enlargement and polycythemia) associated with testosterone administration. 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone; 17beta-TBOH), a synthetic analog of testosterone, may be capable of inducing SARM-like effects as it binds to androgen receptors (ARs) with approximately three times the affinity of testosterone and has been shown to augment skeletal muscle mass and bone growth and reduce adiposity in a variety of mammalian species. In addition to its direct actions through ARs, 17beta-TBOH may also exert anabolic effects by altering the action of endogenous growth factors or inhibiting the action of glucocorticoids. Compared to testosterone, 17beta-TBOH appears to induce less growth in androgen-sensitive organs which highly express the 5alpha reductase enzyme (e.g., prostate tissue and accessory sex organs). The reduced androgenic effects result from the fact that 17beta-TBOH is metabolized to less potent androgens in vivo; while testosterone undergoes tissue-specific biotransformation to more potent steroids, dihydrotestosterone and 17beta-estradiol, via the 5alpha-reductase and aromatase enzymes, respectively. Thus the metabolism of 17beta-TBOH provides a basis for future research evaluating its safety and efficacy as a means of combating muscle and bone wasting conditions, obesity, and/or androgen insensitivity syndromes in humans, similar to that of other SARMs which are currently in development.
Published by Elsevier Inc.
PMID: 20138077 DOI: 10.1016/j.steroids.2010.01.019
[Indexed for MEDLINE]
He doesn't have a real name yet, but if it is up to the important men of Merck / Schering-Plow Pharma , then it will be on the market in the near future: the anabolic steroid MK-0773. You can see his structural formula below.
In fact, experts expected that the S4 already available through websites would conquer the medical market for muscle, bone and libido enhancers. Or else a chemical brother of S4 that comes from the same tube: the GTx company. However, developments in the market for new steroids suggest that the era of steroids has not yet ended.
GTx was founded by the chemists who were the first to make new steroids without a steroid structure. SARMs are called those things: they interact with the androgen receptor, but have hardly any androgenic effects.
GTx collaborated with Merck, but when Merck merged with Schering-Plow, that collaboration ended. If you rely on press releases, GTx will now continue on its own with its own SARMs. [tradingmarkets.com June 11, 2009] [Offline] Merck, who is led by new bosses, has taken up research into the old-fashioned steroids again.
Well, anabolic steroids have a bad name. That is why the Merck researchers pretend that their MK-0773 is also a SARM. SARM is simply a more positive term than 'anabolic steroid'. You can't blame the Mercenaries. Even trenbolone has SARM-like properties, some researchers claim in recent reviews. [Steroids. 2010 Jun; 75 (6): 377-89.]
In the Journal of Biological Chemistry, researchers from Merck Research Laboratories publish results of trials with MK-0773 on rats. They still had the 4-aza-steroid somewhere. The researchers discovered that the thing had an interesting effect when they subjected a whole batch of compounds to a new test. That test determines whether compounds could do fun things with the androgen receptor without causing too many side effects.
DHT, the researchers discovered, binds to the androgen receptor 6 times better than MK-0773. The killeranabolic methyl trenbolone even binds to the androgen receptor 8 times better than MK-0773.
Nevertheless, MK-0773 increases the lean body mass in rats without testes. Although you need more mg / kg for this than if you use DHT, the androgenic side effects - measured by the effect on the seminal vesicles - do not represent anything.


Lowering cholesterol did MK-0773 to a lesser extent than DHT.

At this moment you will find 1 publication about the new drug on PubMed, but Merck has now completed 3 human trials with oral versions of the drug. [clinicaltrials.gov] This also includes trials that compare MK-0773 with good old testosterone. We postpone our assessment of MK-0773 until those studies are published.
Tissue selectivity and potential clinical applications of trenbolone (17beta-hydroxyestra-4,9,11-trien-3-one): A potent anabolic steroid with reduced androgenic and estrogenic activity.
Yarrow JF1, McCoy SC, Borst SE.
Author information
1Geriatric Research, Education & Clinical Center, VA Medical Center, Gainesville, FL 32608, United States. [email protected]
Abstract
Recently, the development of selective androgen receptor modulators (SARMs) has been suggested as a means of combating the deleterious catabolic effects of hypogonadism, especially in skeletal muscle and bone, without inducing the undesirable androgenic effects (e.g., prostate enlargement and polycythemia) associated with testosterone administration. 17beta-Hydroxyestra-4,9,11-trien-3-one (trenbolone; 17beta-TBOH), a synthetic analog of testosterone, may be capable of inducing SARM-like effects as it binds to androgen receptors (ARs) with approximately three times the affinity of testosterone and has been shown to augment skeletal muscle mass and bone growth and reduce adiposity in a variety of mammalian species. In addition to its direct actions through ARs, 17beta-TBOH may also exert anabolic effects by altering the action of endogenous growth factors or inhibiting the action of glucocorticoids. Compared to testosterone, 17beta-TBOH appears to induce less growth in androgen-sensitive organs which highly express the 5alpha reductase enzyme (e.g., prostate tissue and accessory sex organs). The reduced androgenic effects result from the fact that 17beta-TBOH is metabolized to less potent androgens in vivo; while testosterone undergoes tissue-specific biotransformation to more potent steroids, dihydrotestosterone and 17beta-estradiol, via the 5alpha-reductase and aromatase enzymes, respectively. Thus the metabolism of 17beta-TBOH provides a basis for future research evaluating its safety and efficacy as a means of combating muscle and bone wasting conditions, obesity, and/or androgen insensitivity syndromes in humans, similar to that of other SARMs which are currently in development.
Published by Elsevier Inc.
PMID: 20138077 DOI: 10.1016/j.steroids.2010.01.019
[Indexed for MEDLINE]




